WHAT IS APOLLO™?
Apollo™ is an amniotic membrane product that is derived from tissue and cells and procured from human amnion. The amniotic membrane is donated by the birth mother after a scheduled cesarean birth.
Our amniotic membrane products are chorion-free, amnion-only, sterile human tissue allografts intended for human use only, to cover and protect a patient’s ocular tissue. Apollo™ is used as a ‘scaffold’ to encourage and enhance ocular tissue repair and regeneration.

TISSUE TRACKING FORM
Apollo amniotic membrane is regulated by FDA Regulations and Joint Commission Standards. Per these regulations and standards, we have to track the tissue distributed to you and store those records for a minimum of 10 years. It is your responsibility, also per these regulations, to provide the end-user tracking information back to our distribution department for post-transplant tissue tracking. Please complete the below tissue tracking with the pertinent practice and patient information and click submit.
WHAT IS AMNIOTIC THERAPY?
The Apollo™ Amniotic Membrane is a natural, safe, and effective solution and wound covering that aids in healing the surface of the eye. Apollo is a human amniotic membrane allograft disc designed for both in-clinic and operating room use in ophthalmic and optometric applications to help facilitate and enhance ocular tissue repair. The innate properties of amniotic membrane make it an ideal product for expedited wound healing in ocular care.
Apollo membranes are processed human amniotic tissue products recovered from live, healthy, pre-screened donors during scheduled cesarean childbirth. Donor selection, tissue recovery, and processing protocols and procedures meet or exceed all applicable industry standards to ensure patient safety. After passing the rigorous donor screening process (details available upon request), the Medical Director deems the tissue to be suitable for transplantation. The tissue is then sterilized using low-dose, low-temperature gamma irradiation and shipped to customers worldwide sterile and ready for use.
Amniotic membrane tissue is immune privileged therefore patient rejection rarely occurs.
Optometrists and Ophthalmologists are using this advanced regenerative technology where and when needed for in-office procedures.
FOR WHAT TYPES OF PROCEDURES IS APOLLO™ USED?**
- Ocular Surface Disorders
- Corneal Epithelial Defects
- Corneal Ulcer
- Pterygium
- Band Keratopathy
- Moderate to severe dry eye
- Bullous Keratopathy
- Chemical/Thermal burns of the ocular surface
- Herpes simplex keratitis
- Contact lens-induced keratopathy or toxic effects from lens-cleaning solutions
- Stevens-Johnson Syndrome
**This is not a complete list. Please contact your clinical account manager for additional information.
BENEFITS OF APOLLO AMNIOTIC MEMBRANES
There are many benefits to using Apollo amniotic membrane. Here are a few:
- The amniotic extracellular matrix composition of collagen, elastin, fibronectin and proteoglycans provide a natural scaffold to facilitate cellular adhesion while assisting cellular migration and proliferation.
- The amniotic tissue reduces inflammation, pain, and scar tissue formation.
- Apollo adheres well to sclera and conjunctiva when placed on the ocular surface.
- Apollo typically incorporates into tissue in 4-5 days.
- Our membrane is simple to use and can be placed either-side down for ease of placement and maximum clinical efficacy. There is no need for glue or sutures, simply place a BCL to cover the membrane during the healing. This provides a comfortable experience for the patient.
- The 5-year shelf life, at room temperature, means no advance ordering or preparation is required. This advantage makes it convenient for use in inpatient, outpatient or clinic settings.
PRODUCTS OFFERED
APOLLO BIOLOGICAL MEMBRANES
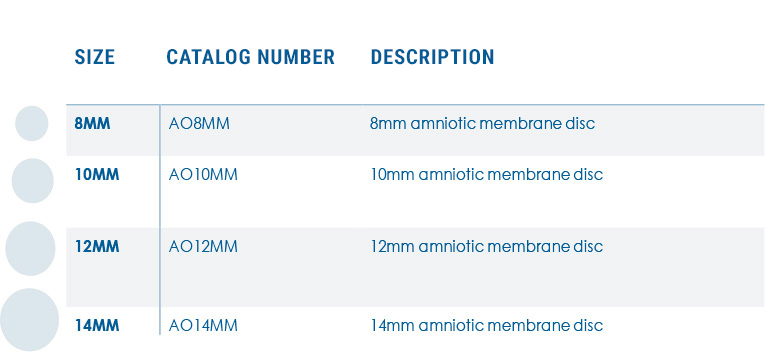
- 8mm Disc
- 10mm Disc
- 12mm Disc
- 14mm Disc
SUPPLIES & EQUIPMENT
- Eye Sponges
- Forceps
- Ophthalmic Burrs
- Hot & Cold Cautery Devices
APOLLO PRODUCT CODES
EASY TO USE AMNIOTIC MEMBRANES
Apollo™ is available in circular sizes for optimal fit. The product can be trimmed to its final desired size while in its dehydrated state. No advance ordering or preparation is required which makes it convenient for use in clinic settings.
Contact your Clinical Account Manager for more information regarding handling and insertion. Guidance for pre- and post- procedure patient instructions are also available upon request.

Numb the patient’s eye


Place the Apollo graft in BCL


Fit BCL on patient’s eye
THINKING OF BECOMING A CLIENT?
Why Work with Atlas Ocular
Atlas Ocular is uniquely positioned to assist you in developing the Apollo amniotic therapy protocol in your practice. The Apollo product was developed after several years of discussions with Ophthalmologists and Optometrists from across the country. These discussions will never end! We believe in continuous learning and seek the latest scientific and medical information to ensure we are always able to provide you with the best support, and best-in-class technology possible.
How We Support Your Practice
- We focus on education and training opportunities for the practitioner.
- Our dedicated team of clinical account managers provide ongoing support, from patient selection through post-procedure visit, including billing guidance.
- Peer-to-Peer with colleagues for clinical questions.
FREQUENTLY ASKED QUESTIONS
Are the procedures covered by insurance?
At this point these procedures are covered by most insurances. While Atlas Ocular does provide general guidance regarding potential reimbursement, coverage, coding and billing information must be confirmed by the individual practitioners as part of their clinical decision making.
CMS and third-party payers do require documentation detailing medical necessity and a description of the procedure.
What is the postoperative surgical period for 65778?
The global surgery period for CPT code 65778 is zero days in 2020. Be certain to confirm the use of any applicable modifiers that may impact reimbursement for unrelated, or planned treatment subsequent to the initial placement.
I have more questions. Where can I get answers?
Your Clinical Account Manager is only a phone call or email away! You may also email customercare@atlasocular.com for further information or click here to send us a contact request.
To place an order, or ask a question regarding an existing order, you may contact customercare@atlasocular.com.
Or, call 833.276.5566. We are happy to answer any questions you may have.
ATLAS OCULAR ADHERES TO THE HIGHEST DEGREE OF SAFETY AND QUALITY STANDARDS
Atlas Ocular is committed to the highest level of safety and quality in the processing of our products. Our placental-derived allografts are recovered from live, healthy donors who are pre-screened during pregnancy and chosen based on stringent donor selections criteria. From aseptic recovery of the tissue at childbirth to timely processing of the allografts, protocols and procedures have been developed to meet or exceed all applicable industry standards for the use of human cellular and tissue-based products.
Atlas Ocular is an FDA registered stocking and storage facility. The Atlas Ocular products are processed for Atlas Ocular by an FDA registered, American Association of Tissue Banks accredited, facility. The Atlas Ocular products are regulated by the FDA under 21 CFR Part 1271 and Section 361 of the Public Health Service Act.
WHAT IS DONOR TESTING?
All potential donors are identified as candidates by their treating OB/GYN. If the healthy donor (mother) gives consent, she is pre-screened by the tissue bank’s Medical Director, a board certified, licensed practicing pathologist and tissue bank specialist. Screenings include a thorough examination of pre-natal medical records and tests results. A comprehensive medical history and behavior risk assessment is obtained from the donor prior to donation, incorporating U.S. Public Health Service guidelines. Discussions with the physician and/or the donor mother are conducted to identify circumstances that may lead to the exclusion of the donor or donate tissue.
The infectious disease screening requirements adhered to by the tissue bank meet or exceed all FDA requirements.
More information available upon request.




